Effect of Superhydrophobic Coating on Corrosion Resistance
Short Description
The main objectives of the proposed project are to 1) design and fabricate, 2) characterize superhydrophobic coatings for enhanced corrosion resistance. A third objective (3) is to evaluate the electrochemical reactions and mechanical performance of superhydrophobic coating.
Project PI
Dr. Chun-Wei Yao, Assistant Professor, Department of Mechanical Engineering
Full Description
Recently, newly surface treatments at micro/nanoscale level have opened a new era in the development of new engineering surfaces for anti-corrosion. In this study, the innovative superhydrophobic surface has been developed. The superhydrophobic surfaces were achieved with wet chemical etching and an immersion method to reduce the complexity of the fabrication process. The superhydrophobicity of the surface was the result of the unique nanostructures and the assembly of low surface energy components, generating a typical contact angle was around 169˚. Furthermore, the corrosion resistance and mechanical properties of the superhydrophobic surface were characterized after immersing surfaces in a 3.5 wt.% NaCl solution. The chemical stability of the superhydrophobic surface in solutions of varying pH values and in the NaCl solution was also evaluated. Immersion measurements and potentiostatic polarization curve measurements revealed that the superhydrophobic surface exhibited an excellent corrosion resistance. An abrasion test and an ultrasound oscillation were conducted to confirm that the surface contained durable superhydrophobic properties. In addition, an atomic force microscope was employed to study the surface mechanical property in the corrosion conditions. This study shows that the resulting superhydrophobic surface exhibit durability, corrosion resistance, and super hydrophobicity which may be presented as an effective solution for protecting metal substrates in various engineering applications.
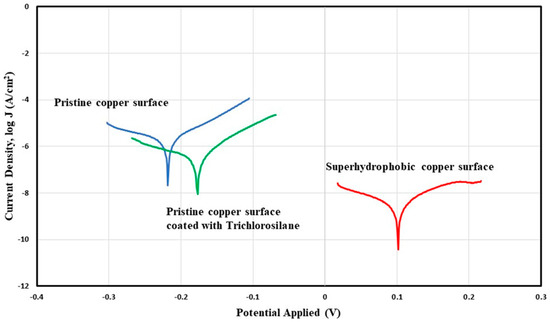
Potentiodynamic polarization curve obtained by using Ag/AgCl electrode as the reference electrode and 3.5 wt % NaCl solution as the electrolyte for pristine copper surface, copper surface coated with Trichlorosilane (reference surface), and superhydrophobic copper surface.
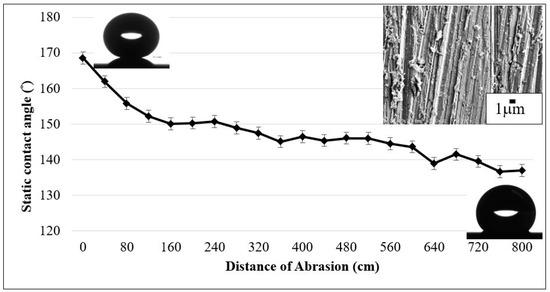
Water contact angles as a result of the abrasion against a 5000 grit sand paper with 7.75 kPa normal load. The inset SEM image (upper right) shows surface morphology after reaching 800 cm of abrasion length.
Funding
This project was funded by Research Enhancement Grants, Lamar University, 2017-2018.
Publications
- C.W. Yao, D. Sebastian, I. Lian, Ö. Günaydın-Şen, R. Clarke, K. Clayton, C.Y. Chen, K. Kharel, Y. Chen, Q. Li, "Corrosion Resistance and Durability of Superhydrophobic Copper Surface in Corrosive NaCl Aqueous Solution," Coatings 8 (2), 70, 2018.
- D. Sebastian, C.W. Yao, I. Lian, "Mechanical Durability of Engineered Superhydrophobic Surfaces for Anti-Corrosion," Coatings 8 (5), 162, 2018.